- 회사소개
- 연구개발
- 사업분야
- IR / PR
- 공지사항
R & D
Herpes Zoster Vaccine
Herpes Zoster Overview
- Varicella Zoster Virus (VZV) is one of eight herpesviruses known to infect humans. It causes chickenpox (varicella), a disease most commonly affecting children, teens, and young adults, and herpes zoster (shingles) in older adults.
- Each year, VZV affects 500,000 persons in the U.S. and 10 million people worldwide. In about 10-20% of cases, VZV reactivates later in life within a person’s body, producing a disease known as herpes zoster or shingles. The incidence of herpes zoster is 1.5 to 3.0 cases per 1,000 persons, and is estimated to occur in up to 20% of individuals during their lifetime. The incidence of herpes zoster in the case of 65 years older is 3.9~11.8 people per year per 1,000 people.

Herpes Zoster Stages
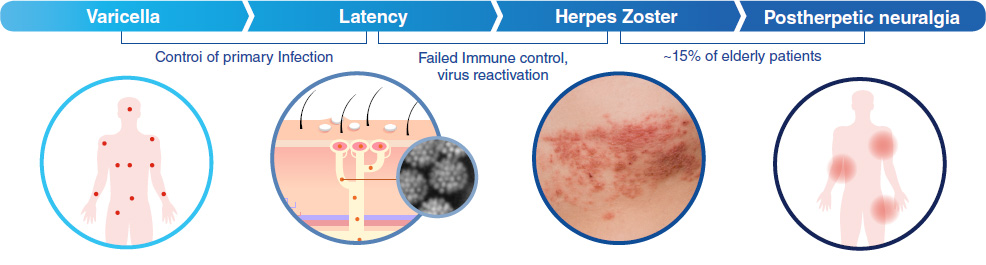
- After the chickenpox infection caused by Varicella zoster virus is over, the virus may remain inactive in nervous system
- It reactivates by poor immune function, the main symptom is painful skin rash with blister in a entire body or localized area
- Facial nerve palsy caused by penetration around the face & meningitis caused by penetrating cranial nerve
- Risk of incidence of intractable pain disease called postherpetic neuralgia(PHN) after treatment
EG-HZ Highlights
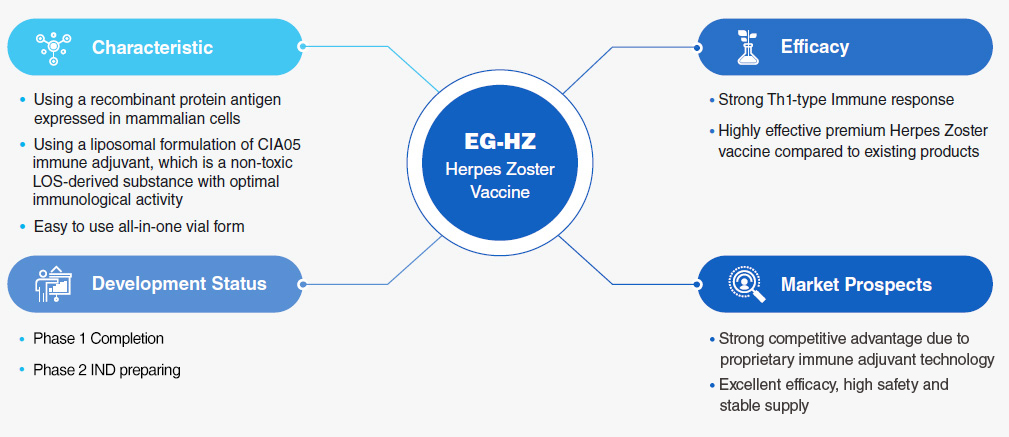
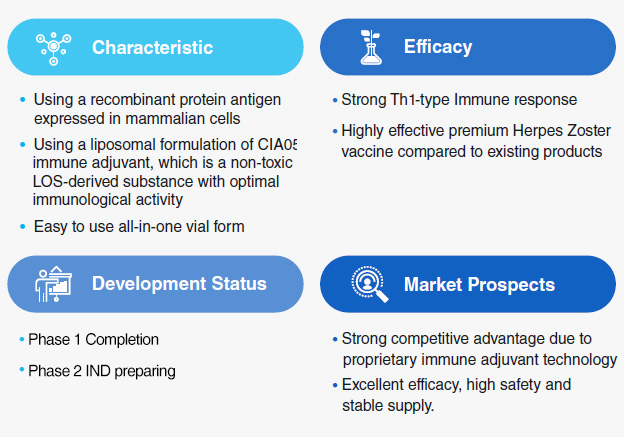
Clinical Trial Status
-
Government
Grant (MOHW
2.7bn. KRW)2014
-
Cell Line
Development
Adjuvant Screening2015
-
MCB Production
Process development2016~2017
-
Pre-Clinical
2018
-
Phase 1 approval

2019.12
-
Phase 1 Completed

2021.06
-
Licensing agreement with BMI Korea

2022
- T. +82-2-322-1687
- F. +82-2-302-4359 / +82-2-324-8059
- 본사 : [07528] 서울특별시 강서구 양천로 401 강서한강자이타워 B동 1211호
- 연구소 : [10551] 경기도 고양시 덕양구 의장로 122-1 2층